Dr Scott Wustenberg D.C, FACNEM, M.Sc. NutMed (Distinction), B.Sc. Chiro, B.Sc. Physiology/Biochemistry
Email: neo@advancerehab.com.au
FREE Full Access of Technical Paper and References
ABSTRACT
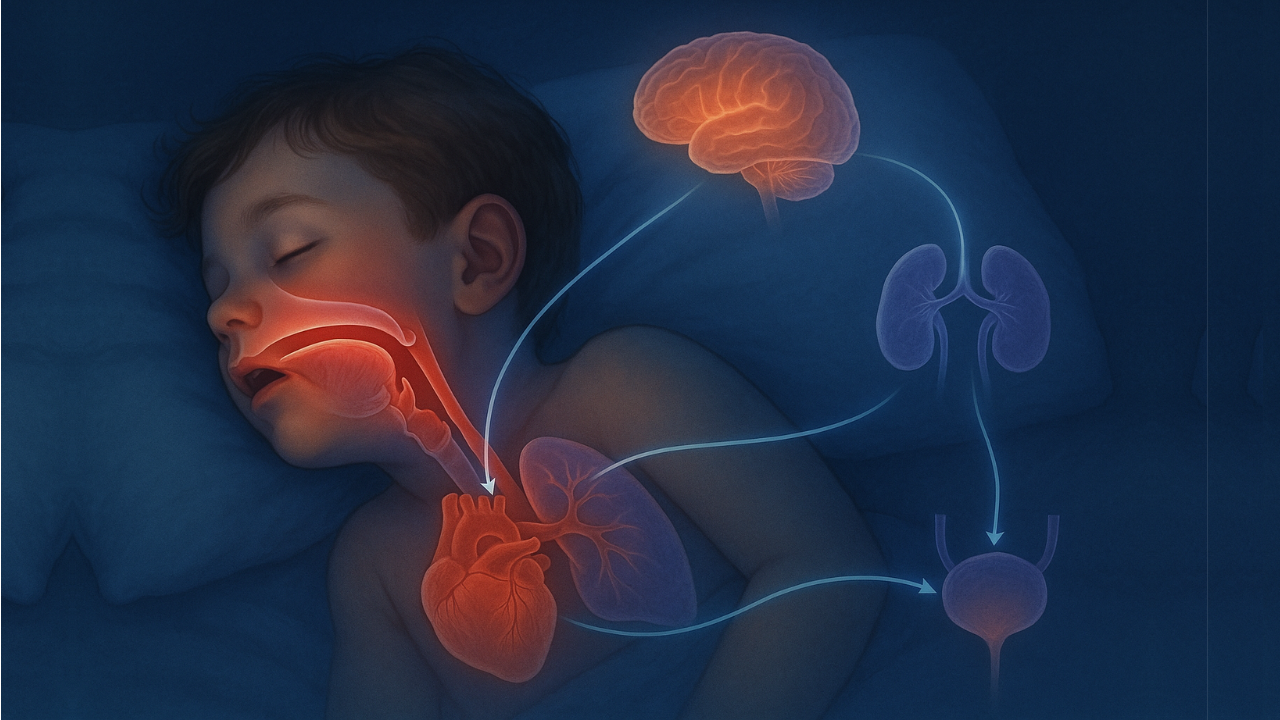
Airway fragmentation—including mouth breathing, airway occlusion, apnoea, and hypopnea—initiates a series of biochemical, physiological, and neurological events that culminate in nocturnal enuresis (bedwetting). These mechanisms involve hormonal dysregulation, sympathetic overactivation, renal dysfunction, altered sleep architecture, detrusor instability, and metabolic disturbances. This paper outlines 10 interconnected pathways linking sleep-disordered breathing (SDB) to bedwetting, with emphasis on natriuretic peptides, antidiuretic hormone disruption, cortisol and catecholamines, RAAS activation, renal hypoxia, bladder physiology, arousal mechanisms, and respiratory alkalosis from mouth breathing.
1. Hormonal Pathway: Natriuretic Peptides (ANP/BNP)
Mechanism
Upper airway obstruction increases inspiratory effort against an occluded airway, generating severe negative intrathoracic pressure (–15 to –17 mmHg). This markedly increases venous return and stretches the atrial and ventricular walls, triggering release of:
- ANP (Atrial Natriuretic Peptide) from the atria
- BNP (Brain/B-type Natriuretic Peptide) from the ventricles
Effect on Kidneys
ANP/BNP:
- Increase sodium and water excretion
- Suppress RAAS
- Reduce ADH secretion
- Increase GFR
Result → Nocturnal polyuria exceeding bladder capacity.
Effect on Blood Pressure
Although ANP/BNP are vasodilatory, this effect is overshadowed by sympathetic surges at the termination of apnoeic events.
Evidence
ANP and BNP levels correlate directly with apnoea severity. Elevated morning BNP is found in children with SDB and enuresis. These markers normalize following adenotonsillectomy, CPAP, or airway-expanding interventions (e.g., TOT release, orthodontics).
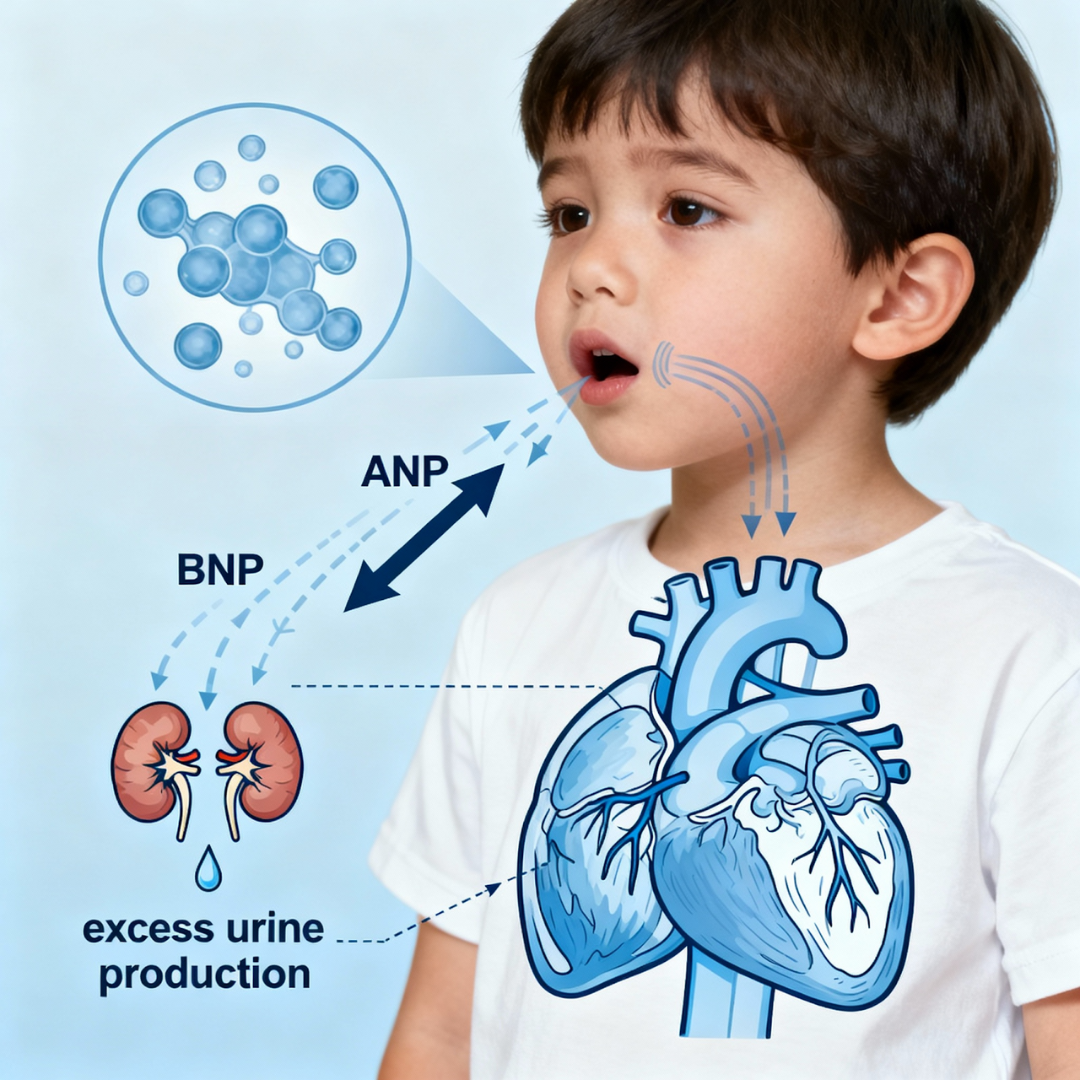
Cardiac stretch mechanism: Airway obstruction causes negative intrathoracic pressure, stretching heart chambers and triggering ANP/BNP release, leading to increased kidney urine production.
2. Hormonal Pathway: Antidiuretic Hormone (ADH/Vasopressin) Suppression
Mechanism
Normal sleep increases ADH to reduce nighttime urine volume. Sleep fragmentation from apnoea/hypopnea disrupts ADH circadian rhythm. Elevated ANP/BNP further suppress ADH release.
Effect on Kidneys
Reduced ADH →
- Impaired water reabsorption
- Dilute nocturnal urine
- Increased urine volume
→ Bladder overfilling
Effect on Blood Pressure
Reduced vasopressin minimally contributes to nocturnal blood pressure variance relative to sympathetic activation.
Evidence
Enuretic children with OSA exhibit absent or blunted nocturnal ADH peaks and show a “non-dipping” nocturnal blood pressure pattern.
3. Hormonal Pathway: Cortisol and Catecholamines (HPA Axis + Sympathoadrenal System)
Mechanism
Hypoxia and repetitive arousals activate:
- HPA axis → cortisol
- Sympathetic nervous system → adrenaline and noradrenaline
This is a survival-based response to airway obstruction.
Effect on Kidneys
Cortisol alters glucose handling, immune signaling, fluid balance, and bladder sensory pathways.
Catecholamines:
- Renal vasoconstriction
- Altered GFR
- Increased sodium reabsorption
- Increased renin release
Children with OSA demonstrate a ~45% increase in 24-hr urinary noradrenaline.
Effect on Blood Pressure
Catecholamine-driven vasoconstriction leads to:
- Increased peripheral resistance
- Tachycardia
- Sustained daytime hypertension
- Heightened chemoreceptor sensitivity
Evidence
Muscle sympathetic nerve activity (MSNA) is significantly elevated in OSA and decreases with CPAP. Catecholamine levels correlate with hypoxemia severity.
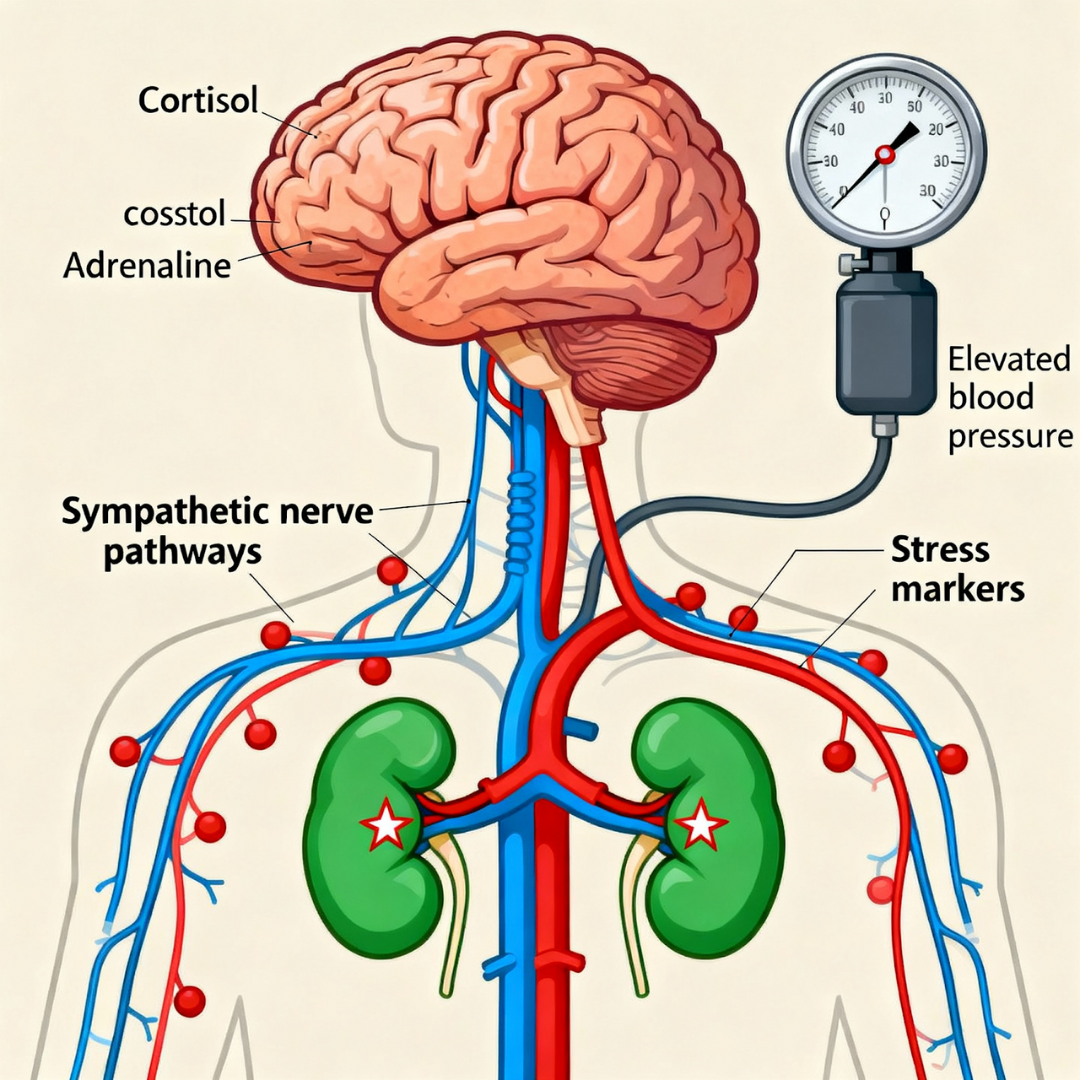
Stress hormone pathway: Hypoxia activates HPA axis and sympathetic nervous system, releasing cortisol and catecholamines that affect kidney function and blood pressure regulation
4. Cardiovascular Pathway: Negative Intrathoracic Pressure Load
Mechanism
Obstruction → futile inspiratory efforts → extreme negative intrathoracic pressure. Results:
- Increased LV transmural pressure (↑ afterload)
- Increased venous return to RV (RV distension)
- Septal shift → impaired LV filling (↓ preload)
- Reduced stroke volume during apnoea
Effect on Kidneys
Increased venous return and atrial stretch → continuous ANP/BNP secretion → nocturnal sodium and water loss.
Effect on Blood Pressure
Initial drop (reduced CO) → rebound sympathetic surge → hypertensive peaks after each apnoea.
Evidence
Intrathoracic pressure swings correlate with ANP rises and urinary cGMP elevation.
5. Renal Pathway: RAAS Activation
Mechanism
Intermittent hypoxia + sympathetic stimulation → ↑ renin → ↑ angiotensin II → ↑ aldosterone.
Effect on Kidneys
Although RAAS normally promotes fluid retention, its actions are overwhelmed by high ANP/BNP, leading to increased sodium excretion.
Effect on Blood Pressure
Angiotensin II → vasoconstriction
Aldosterone → fluid retention
→ Hypertension, especially resistant hypertension associated with OSA.
Evidence
OSA patients show higher plasma renin activity, aldosterone, and angiotensin II. CPAP partially reduces RAAS activation.
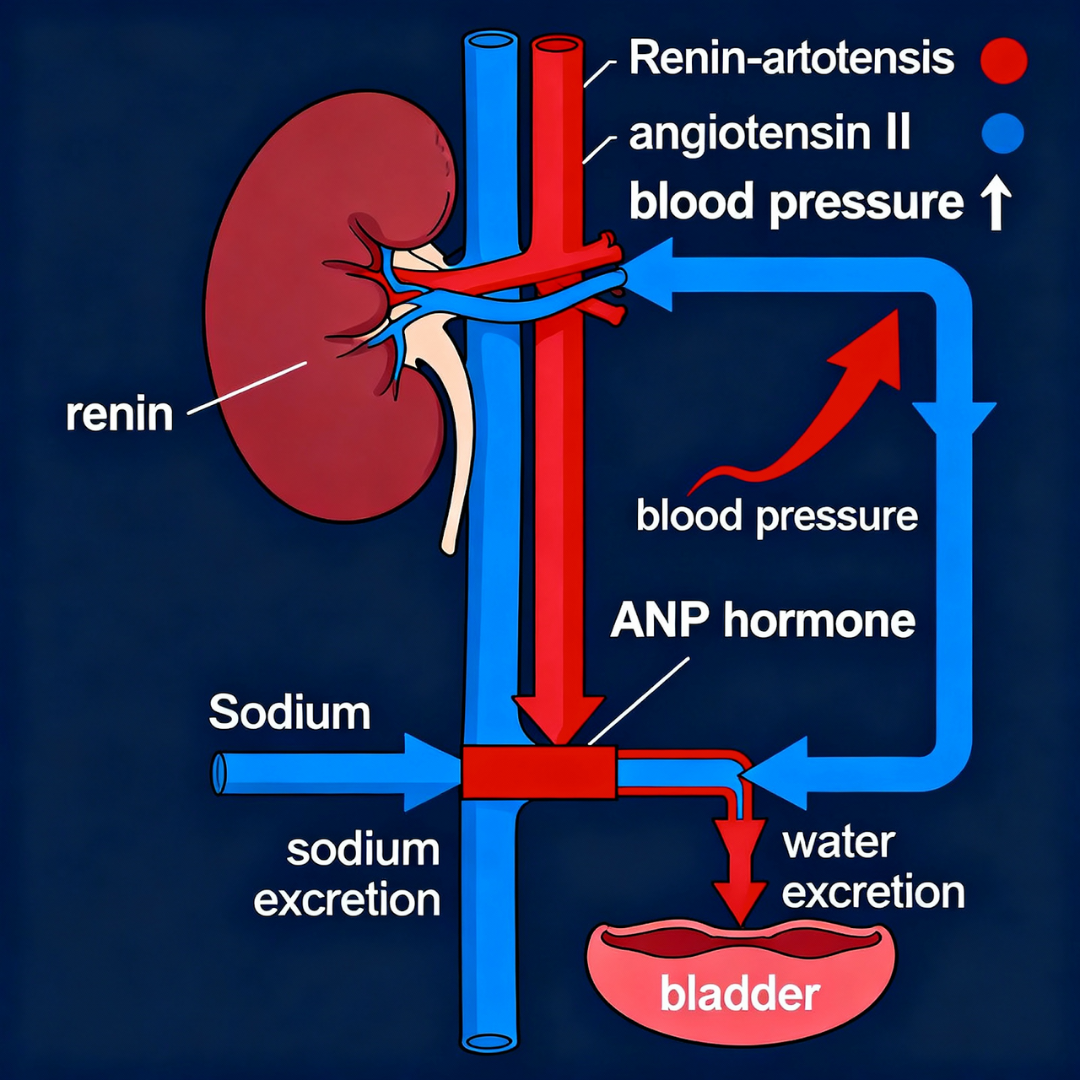
Renal hormonal pathway: RAAS activation increases blood pressure while ANP/BNP override this system, promoting excessive sodium and water excretion at night
6. Renal Pathway: Direct Hypoxic Damage to Kidney Tissue
Mechanism
Intermittent hypoxia triggers:
- Reactive oxygen species (ROS)
- HIF activation
- Inflammatory cytokine release
- Renal sympathetic nerve activation
Effect on Kidneys
- Tubular dysfunction
- Impaired urine concentration
- Tubulointerstitial injury
- Reduced renal perfusion
→ Increased nocturnal urine output and sodium loss.
Effect on Blood Pressure
Renal hypoxia contributes to:
- Endothelial dysfunction
- Impaired pressure-natriuresis
- Further sympathetic elevation
- Chronic kidney disease progression
7. Neurological Pathway: Elevated Arousal Threshold
Mechanism
Chronic sleep fragmentation increases homeostatic sleep pressure → higher arousal threshold.
Children enter deep sleep more persistently and struggle to wake even with bladder distension.
Effect on Bladder/Kidneys
- Reduced sensitivity to bladder signals
- Failure to transition from deep sleep when detrusor contraction occurs
→ Enuresis during NREM transitions.
Evidence
Polysomnography shows:
- Higher CAP rates in N3
- Reduced A2/A3 arousals
Enuretic children exhibit difficulty waking despite bladder signaling.
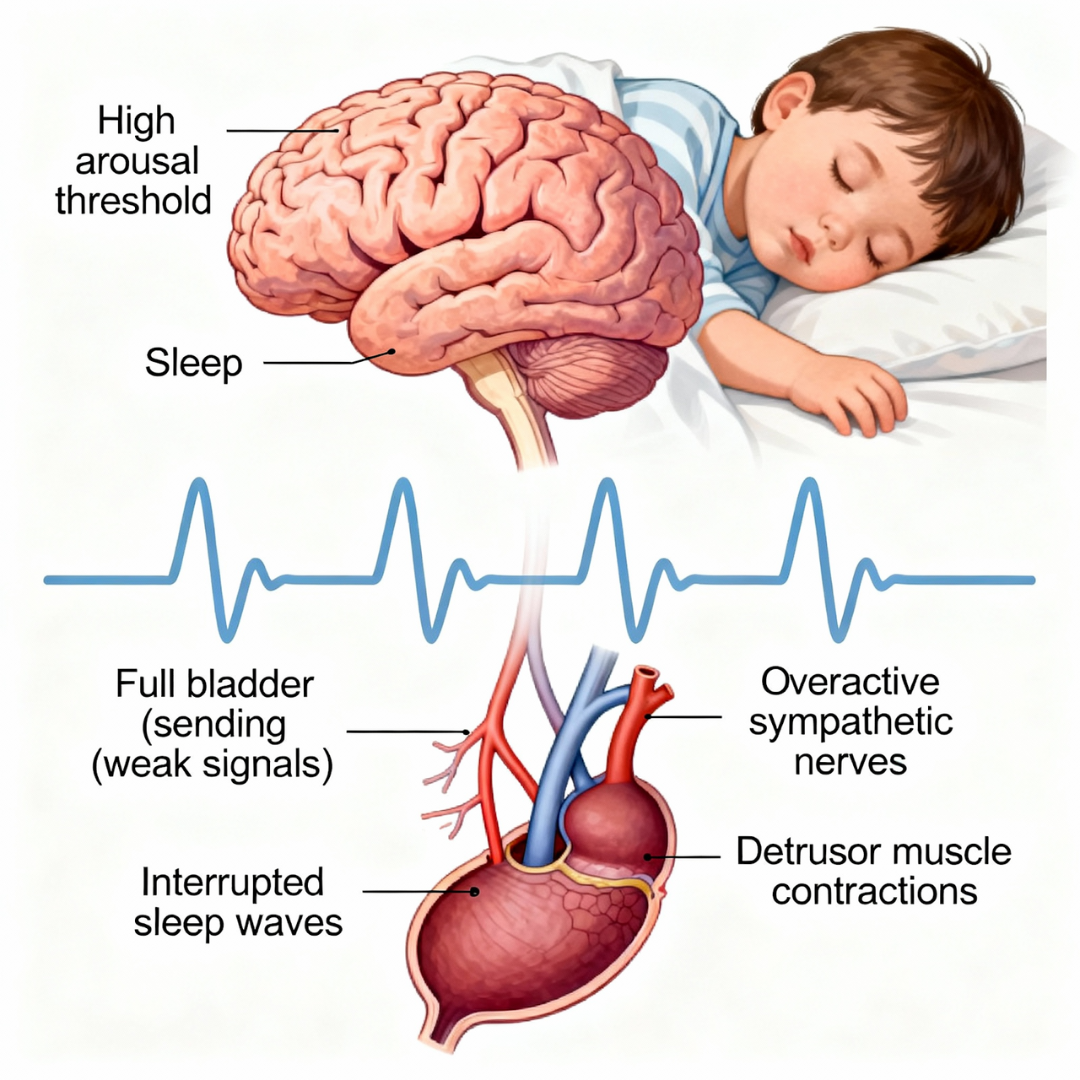
Neurological arousal mechanism: Sleep fragmentation elevates arousal threshold while sympathetic overactivation causes bladder overactivity, preventing the child from waking to bladder signals
8. Neurological Pathway: Sympathetic Overactivation
Mechanism
Hypoxia + arousals + respiratory effort → chronic sympathetic overactivation.
Effect on Kidneys
- Altered renal blood flow
- Increased renin
- Sodium retention patterns modified
- Impaired bladder innervation balance
Effect on Bladder
Sympathetic overdrive contributes to:
- Detrusor overactivity
- Bladder hypersensitivity
- Increased urgency and reduced capacity
Effect on Blood Pressure
Central driver of hypertensive patterns in OSA.
9. Bladder Pathway: Detrusor Overactivity and Instability
Mechanism
Intermittent hypoxia induces oxidative damage in bladder smooth muscle:
- Mitochondrial dysfunction
- ROS accumulation
- Altered calcium handling
- Smooth muscle structural change
Effect on Bladder
- Premature detrusor contractions
- Reduced functional capacity
- Bladder wall thickening
→ Overactive bladder phenotype.
Clinical Link to Enuresis
With an elevated arousal threshold, premature detrusor contraction leads to involuntary nocturnal voiding.
Evidence
Severity of OSA correlates with:
- Bladder wall thickness
- Overactive bladder symptom scores
- Time spent below 90% oxygen saturation
10. Metabolic Pathway: Mouth Breathing and Respiratory Alkalosis
Mechanism
Chronic mouth breathing → CO₂ over-exhalation → hypocapnia → respiratory alkalosis
Effect on Kidneys
To compensate, kidneys excrete:
- Bicarbonate
- Minerals including magnesium, sodium, phosphorus, calcium
Systemic Consequences
- Reduced tissue oxygen delivery (left-shifted oxyhemoglobin curve)
- Impaired neuromuscular function
- Reduced cellular ATP production
- Increased vulnerability of bladder and kidney tissues
Evidence
Mouth breathing increases net water and energy loss by 42%. Blood-gas shifts reduce oxygen unloading to tissues despite normal oxygen saturation values.
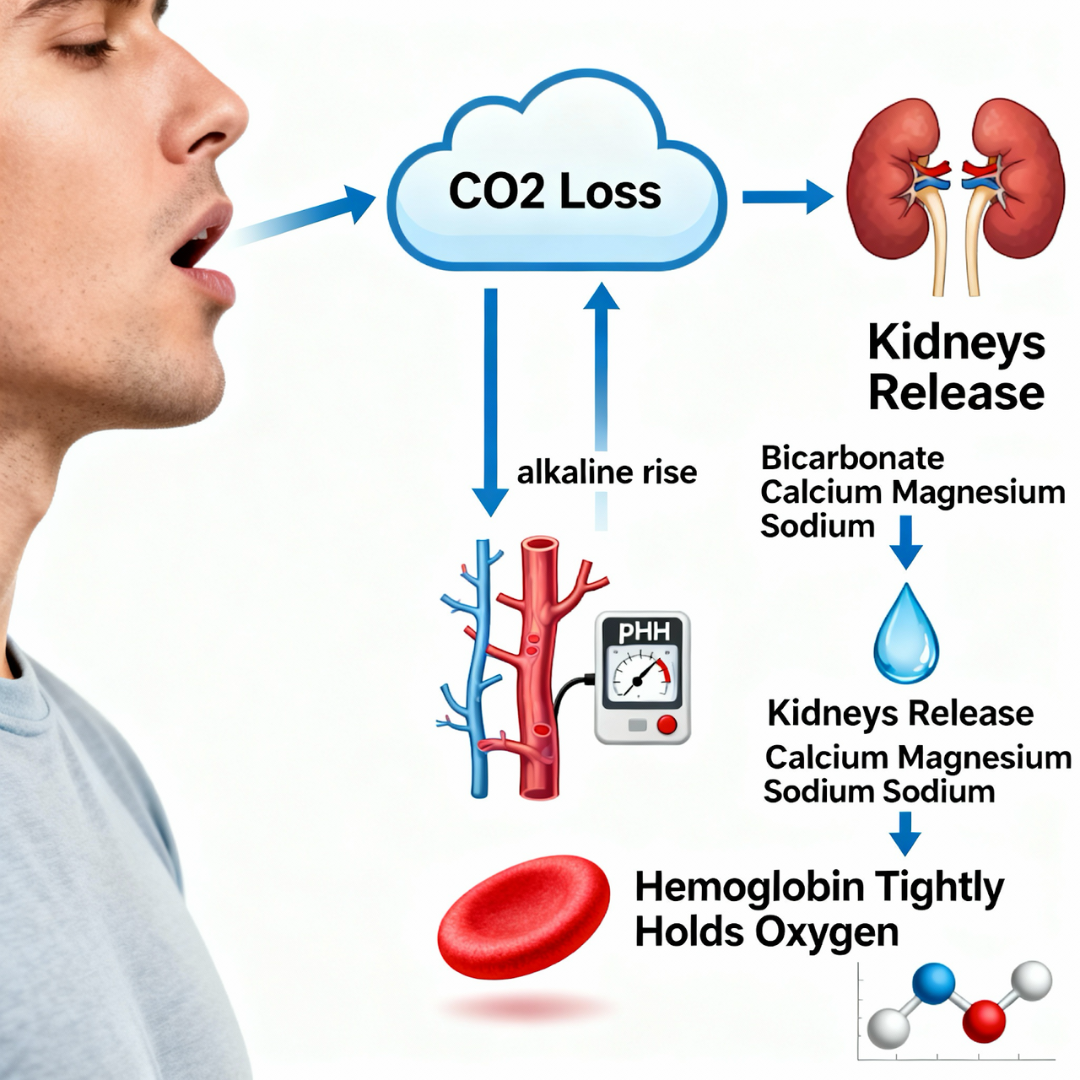
Respiratory alkalosis mechanism: Mouth breathing causes CO2 loss and alkaline blood pH, triggering renal compensation with mineral loss and impaired oxygen delivery to tissues
Integrated Mechanistic Cascade
Airway obstruction →
→ Negative intrathoracic pressure → ANP/BNP release → nocturnal diuresis
→ Hypoxia → cortisol + catecholamines → hypertension + renal changes
Sleep fragmentation → ADH suppression + high arousal threshold
Renal hypoxia → impaired concentrating ability
Bladder oxidative stress → detrusor overactivity
Mouth breathing → alkalosis → mineral loss → impaired neuromuscular control
Combined outcome:
- Nocturnal polyuria
- Bladder overactivity
- Failure to awaken to bladder signals
→ Nocturnal enuresis

Complete integrated pathway: All five major mechanisms (cardiac, stress, renal-hormonal, neurological, and metabolic) work together to cause nocturnal enuresis in children with airway obstruction
Clinical Implications
Treatment of airway obstruction consistently improves bedwetting through:
- Reduced negative intrathoracic pressure
- Lower ANP/BNP secretion
- Restoration of ADH rhythm
- Reduced sympathetic tone and catecholamines
- Improved RAAS balance
- Normalization of blood pressure patterns
- Improved sleep architecture
- Renal and bladder tissue recovery
Adenotonsillectomy improves enuresis in 68–85% of children with OSA. CPAP in adults reduces nocturia and bedwetting episodes.
Emerging evidence suggests tethered oral tissue release and airway-expansion orthodontics may provide similar improvements by restoring airway patency.
Bedwetting associated with airway obstruction is therefore a physiological disorder, not a behavioural or developmental failure.
FREE Full Access of Technical Paper and References
Comments are closed.
